-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
In the following structure, the double bonds are marked as I, II, III and IV  Geometrical isomerism is not possible at site (s) :
Geometrical isomerism is not possible at site (s) :
Option: 1 III
Option: 2 I
Option: 3 I and II
Option: 4 III and IV
Geometrical isomerism is not possible at Site I as two identical methyl groups are attached to the same carbon bearing the double bond.
Hence, the answer is Option (2)
View Full Answer(1)The most abundant elements by mass in the body of a healthy human adult are : Oxygen (61.4%); Carbon (22.9%), Hydrogen (10.0%); and Nitrogen (2.6%). The weight (in kg) which a 75 kg person would gain if all atoms are replaced by
atoms is :
Option: 1 7.5
Option: 2 10
Option: 3 15
Option: 4 37.5
Given that
Mass of the person = 75 kg
Mass of 1H1 present in person = 10% of 75 kg = 7.5 kg
Since Mass of 1H2 is double the Mass of 1H1
So, Mass of 1H2 will be in person = 2 X 7.5 kg =15 kg
Thus, increase in weight = 15 - 7.5 = 7.5 kg
Therefore, Option (1) is correct
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
Which of the following compounds will show the maximum 'enol' content?
Option: 1
Option: 2
Option: 3
Option: 4
Difinition of Tautomerism -
Tautomers are isomers of a compound that differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism.

The stability of enol depends on the factors
(1) Resonance
(2) Hydrogen-bonding
(3) Hyperconjugation
(4) Hydrogen which is removed from - carbon should be acidic in Nature for enol formation.
CH2 is present between 2- electron-withdrawing group, It is having acid 'H'
Over the resonance effect decreases because of cross conjugation. So the stability of the enol form decreases.
The two -dicarbonyl compounds have a higher enol content than the two mono carbonyl compounds because hydrogen bonding and conjugation stabilize their enols. The enol content in C (a mono aldehyde) is higher than D because of the reasons outlined above.
Therefore, option (2) is correct.
View Full Answer(1)- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
Among the compounds, A and B with molecular formula A is having higher boiling point than B. The possible structures of A and B are :
Option: 1
Option: 2
Option: 3
Option: 4
In (A), extensive inter-molecular H-bonding is possible while in (B) there is no Inter-molecular H-bonding.
Option 4 has this type of arrangement that follows the above conditions.
Therefore, Option(4) is correct.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
The increasing order of values of the following compounds is :

Option: 1 II<IV<III<I
Option: 2 II<I<III<IV
Option: 3I<II<III<IV
Option: 4 I<II<IV<III

III contains -M and -I group, hence it is the least basic.
IV contains -I group
I contains +M group and is more basic than II.
Thus order of strength: I > II > IV > III.
Order of : I < II < IV < III.
Therefore, Option(4) is correct.
View Full Answer(1)- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
The correct order of statbility for the following alkoxides is:
Option: 1 C > A > B
Option: 2 C > B > A
Option: 3 B > A > C
Option: 4 B > C > A
When a negative charge is delocalised with an electron-withdrawing group like (NO2) then stability increases.
(A) The negative charge is localised
(B) The negative charge is delocalised with the carbon of the alkene
(C) Negative charge is delocalised with NO2 group
So, the order will be
C > B > A
Therefore, Option(2) is correct.
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
The ammonia released on quantitative reaction of 0.6g, urea
with sodium hydroxide
can be neutralised by:
Option: 1 200 ml of 0.2 N HCl
Option: 2200 ml of 0.4 N HCl
Option: 3100 ml of 0.1N HCl
Option: 4100 ml of 0.2N HCl
2 × mole of Urea = mole of ........(1)
mole of = mole of
........(2)
mole of HCl = 2 × mole of Urea
mole of HCl = ...(i)
[We know , mole = M X V = N X n X V]
...as (i).
Therefore, Option(4) is correct.
View Full Answer(1)- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
The IUPAC name of the following compound is :

Option: 1 2- nitro-4-hydroxymethyl-5 -aminobenzaldehyde
Option: 2 3-amino-4-hydroxymethyl-2-nitrobenzaldehyde
Option: 3 5-amino-4-hydroxymethyl-2-nitrobenzaldehyde
Option: 4 4-amino-2-formyl-5-hydroxymethyl nitrobenzene
Stepwise -
1. CHO comes first in the seniority table.
2. Numbering
Clockwise - 2+4+5 = 11 Minimum (Correct)
AnitClockwise - 3+4+6 = 13

3. Write all substitute Prefix Name with Number
5-amino
4-hydroxymethyl
2-nitro
4. Main group SUffix Name
benzaldehyde
5. write the Prefix name with Number in alphabetical order.
The IUPAC name:
5-amino-4-hydroxymethyl-2-nitrobenzaldehyde
Therefore, the correct option is (3).
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The average molar mass of chlorine is 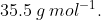 The ratio of
The ratio of 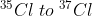 in naturally occurring chlorine is close to :
in naturally occurring chlorine is close to :
Option: 1 4:1
Option: 2 3:1
Option: 3 2:1
Option: 4 1:1
Given,
The average molar mass of chlorine is
Let , the ratio of in naturally occurring chlorine is close to x : y
Now, we know
So,
Therefore, the correct option is (2).
View Full Answer(1)
The minimum number of moles of O2 required for complete combustion of 1 mole of propane and 2 moles of butane is ______
Combustion reaction of 1 mole of propane and 2 moles of butane-

So, Total required mol of O2 = 5 + 13 = 18.
Ans = 18
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE







